FierceBiotech published the top 15 biotech VC deals of 2010 last week, measured by dollars invested. Since they noted an overall uptick in investments in 2010, it seemed like a worthwhile time to look back. Here’s what U.S. VC investment in biopharma and medical devices looked like from 2007 to 2010 (normalized to 2007 levels):
Not unexpectedly, a huge decline between 2007 and 2009, though not as big as the overall decline in VC investments. Here’s the really interesting part — the average amount invested (±1σ) among the top 15 deals each year:
Remarkably stable. Even during a period of steeply declining investment there will be standouts that generate real excitement, proving that as FierceBiotech said in 2008 ”[g]ood science will attract funding in any market.”
It’s not a surprise that good ideas always get some funding, but why do the top investees always attract the same amount? The price of admission to the top 15 between 2007 and 2010 has ranged only between $39 and $42 million.
It must be that (once a concept reaches a certain stage) the amount of money needed to really propel a life sciences company to success is constant — apparently an average of $50 – $60 million — and recognizing that, VCs will fund their best prospects to that level even at the expense of other investments. So the next time you’re contemplating a $10 million C round, keep in mind that you’re more than two standard deviations off the mean investment made when VCs really mean it. It’s an interesting idea the other way too: Pacific Biosciences, which IPO’d in the middle of its range at $16/share last October, was the top deal twice in four years (including the +2.4σ variant of $109m in 2010). It’s currently trading at $15.74, giving it a market cap of $831.43 million, just over double the reported $370 million of VC that it raised prior to the IPO.
Check out FierceBiotech’s list of the top VC investments from 2010, 2009, 2008 and 2007 and apply your own 20:20 hindsight to your heart’s content. Also, keep your fingers crossed that a 3% increase stops feeling like such a victory when we see the 2011 data.
Re-posted from the Cross-Border Biotech Blog
Jeremy Grushcow is a Foreign Legal Consultant practising corporate law at Ogilvy Renault LLP. He has a Ph.D. in Molecular Genetics and Cell Biology. His practice focuses on life science and technology companies.
![]() The RIC blog is designed as a showcase for entrepreneurs and innovation. Our guest bloggers pro vide a wealth of information based on their personal experiences. Visit RIC Centre for more information on how RIC can accelerate your ideas to market.
The RIC blog is designed as a showcase for entrepreneurs and innovation. Our guest bloggers pro vide a wealth of information based on their personal experiences. Visit RIC Centre for more information on how RIC can accelerate your ideas to market.










































 By Jeremy Grushcow
By Jeremy Grushcow











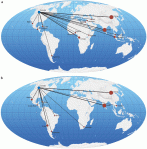 The figure from the paper on the left shows the geography of, and rationale for, the collaborations. Part “a” shows marketing and distribution collaborations, and part “b” shows those involving an R&D component.
The figure from the paper on the left shows the geography of, and rationale for, the collaborations. Part “a” shows marketing and distribution collaborations, and part “b” shows those involving an R&D component.